User:EvilJackCarver/research notes/Guide to Atmospherics: Difference between revisions
(create, my fingers hurt) |
(→Definitions used in thsi guide: i cannot spell at 1 AM) |
||
| Line 6: | Line 6: | ||
There are many different parts to Atmospherics, again, mostly theoretical. For simplicity's sake, this page will only cover theoreticals. And, for the love of Nanotrasen... Don't just immediately go for the fireaxe. | There are many different parts to Atmospherics, again, mostly theoretical. For simplicity's sake, this page will only cover theoreticals. And, for the love of Nanotrasen... Don't just immediately go for the fireaxe. | ||
==Definitions used in | ==Definitions used in this guide== | ||
* '''20/80''' - Airmix ratio for your standard airmix ("20% oxygen, 80% filler gas") | * '''20/80''' - Airmix ratio for your standard airmix ("20% oxygen, 80% filler gas") | ||
Revision as of 20:24, 17 January 2017
DISCLAIMER: The information here refers to /tg/ code in general, and may not account for FTL13-specific quirks or oddities.
Welcome to Atmospherics. As I've said many a time before in-game, Atmos is a "whole lot of theory, and a little bit of piping" - and it really is. Knowing how to do the piping is one thing, but knowing the theoreticals behind why 100% oxygen environments is bad, why the contaminated mode on the air scrubbers is a godsend, and why nitrogen is a good fire suppressant is what separates the atmos techs from the greyshirts wearing yellow-and-blue.
There are many different parts to Atmospherics, again, mostly theoretical. For simplicity's sake, this page will only cover theoreticals. And, for the love of Nanotrasen... Don't just immediately go for the fireaxe.
Definitions used in this guide
- 20/80 - Airmix ratio for your standard airmix ("20% oxygen, 80% filler gas")
- Airmix - As the name implies, the mix for the breathable air. Used in this guide as "O2/filler gas". See 20/80 above.
- Content - The amount of gas, i.e. in a mix. Abbreviated in maths here as C.
- Filler or Filler gas - The other gas in airmix that is not oxygen, generally N2.
- Oxidizer - Critical component for a fire, in this game it will be O2.
- Specific heat - The amount of energy required to heat a specific amount of gas by 1 degree Celsius.
Air Alarms
These little devices are your best friend when you're an Atmospherics technician: If something is off, it will flash its red light insistently and post a warning on the atmospherics and station monitoring consoles. You control the scrubbers and vents from these.
At the top of your window, are the raw numbers - anything in orange is out of ordinary but still safe. Anything in red needs attention. Below the ambient temperature and pressure is the gas composition immediately at the tile in front of the air alarm. For the most part, you are only going to want to see oxygen, nitrogen, and less than 0.03% of carbon dioxide.
Vent Controls
For every vent, you have 3 settings:
- Power - Self-explanatory. Master vent power switch.
- Pressure Regulator - The pressure regulator selection. Internal and External do two very different things and should NOT be confused.
- Internal - The vent will try to push as much air out as possible to maintain a certain in-pipe target pressure. If the target pressure is below the measured pressure, the vent is on. Rarely used.
- External - The vent will try to maintain a certain outside-vent target pressure, measured on the square the vent is on. If the target pressure is above the measured pressure, the vent is on.
- Target Pressure - The pressure the vent will try to maintain.
Scrubber Controls
For every scrubber, you have 4 settings:
- Power - Again, self-explanatory. Master scrubber power switch.
- Mode - The scrubber mode selection. Much like the vent's pressure regulator, the two modes do two very different things. Unlike the pressure regulator, this much is pretty obvious at first glance.
- Scrubbing - The main mode. The scrubber will siphon out all the gases it encounters in the 'filters' selection below.
- Siphoning - The scrubber will siphon out ALL gases it enounters.
- Range - Self-explanatory, how far the scrubber will reach.
- Filters - Which gases the scrubber in scrub mode should get out of the air.
Operating Modes
You have several operating modes, to suit the needed task at hand.
- Filtering - This is the default mode. The scrubbers filter out CO2, and the vents are set at 101.3 kPa target pressure.
- Contaminated - The scrubbers are set to filter out all contaminants, and the vents are set at 101.3 kPa target pressure. This is the best mode to be in, as it filters all contaminants out quickly, and a locked air alarm can be set to filter out all the nasties and re-locked in about ten seconds with practice.
- Draught - The scrubbers are set to siphon, and the vents are set to output at 202.7 kPa target pressure to compensate for the air being sucked out of the room. This mode is useful for temperature-related issues, but the temperature increases slowly.
- Refill - The scrubbers are set to filter, and the vents are set at 303.9 kPa target pressure. Useful for refilling large areas, dangerous otherwise. Keep a close eye on the pressure if you use this mode, or you can easily overpressurise an area. Rarely used.
- Cycle - This mode is unique: Pressing it sets the mode to panic siphon (see below) until the air pressure is negligible. Once the pressure is below a certain single-digit level, the air alarm automatically sets the mode back to Filtering. This mode is useful for temperature-related issues, if you can keep idiots from barging in while it works, as well as large-scale contamination. Rarely used.
- Siphon - This mode removes air from the room. The scrubbers are set to siphon, and the vents are off. Useful for dealing with overpressurisations. Rarely used.
- Panic Siphon - Like syphon, removes air from the room, but quickly. The difference between it and siphon are the scrubbers are set to siphon on expanded mode, which does it much faster. Useful for extreme cases of overpressurisation, as well as salvaging air from a breached room. Also useful for firefighting, with the caveat that it will severely choke up the waste line with hot gas.
Pressurisation
Repressurising areas is your main job as an Atmospherics Technician. It is also one of the most important on the ship. In order to survive comfortably, humans (used loosely) need approximately 101.3 kilopascals (kPa) of ambient pressure. The number is much much lower, however; the minimum pressure needed to survive sans hardsuit is about 20.27 kPa. Also of note, canisters pressurise the tile it's on, which spreads to adjacent tiles in a plus-shaped fashion - think Minecraft fluid physics.
The above... isn't very relevant. What is relevant, however, is this: Humans need 16 kPa of oxygen to breathe. This means at standard 20/80 airmix, humans need 80 kPa of ambient pressure to survive without internals. The math can be summed up as follows:
16 ÷ C
where C is the decimal form of the oxygen content (in the case of 20/80, 20% or 0.2).
As the above maths imply, a higher oxygen content means you only need to pressurise a room to a lower pressure for humans to be able to breathe. While this may make it sound like setting the oxygen content up to 50% is a good idea, in practice it is not.
For starters, you will lose a LOT of your oxygen to space if you get a breach in a 100% oxygen environment, whereas if you have a 20/80 environment, you are only losing 1 kPa of oxygen for every 4 kPa of filler gas. This is important to remember, because the oxygen tank in Atmosia has a very finite amount of O2 in it. This is why Atmospherics are regarded as the "first in, last out" - in the event of a breach, you are the first in to turn off the vents near it to reduce space wind and waste, and last out of it after repressurising it and turning the vents back on.
100% oxygen environments are bad for one other major reason...
Firefighting
So some greyshirt opened a can of plasma in the main hall. See that firesuit in the atmospherics locker? Guess what that means... That's right, you're the station's ghetto fireman. Always wear your internals when fighting fires. Always.
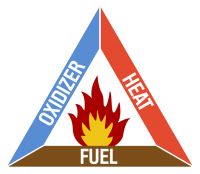
In order to fight fires, you must first understand fires. Much like in the real world, in order for a fire to survive, it needs three things: Oxidizer, fuel, and heat. These three things are known as the Fire Triangle, because a fire cannot start without all of them, and a blaze in progress will die if one is removed. This is another reason the airmix is so lean: A fire will burn out the oxidizer in a, say 3-by-3 room much faster if it's only 20% oxygen rather than 100% oxygen. More oxygen means more oxidizer.
SIDE NOTE: While there is no other oxidizer besides oxygen in /tg/ code at the moment, the decision was made to use the word 'oxidizer' instead of 'oxygen' in the event another oxidizer was added later, and as a nod to the fact that oxygen isn't strictly required for a fire in real life (see: NASA space shuttle rockets).
Another note to take is specific heats: A room of pure oxygen will heat faster than a room of pure nitrous oxide. Oxygen and nitrogen share the lowest specific heat, but nitrogen is used as the filler gas because the rest have adverse effects when breathed. The specific heats known, per the Guide to Atmospherics, are as follows:
- O2: 20
- N2: 20
- CO2: 30
- N2O: 40
- Plasma: 200
This means a room of 100% oxygen will heat ten times slower than a room of 100% oxygen.
So let's go back to our scenario, where the greyshirt opened a can of plasma in the main hall. Remember: A fire cannot exist without the three elements of the fire triangle - you can deprive the potential (or raging) fire of its fuel (siphon the plasma out), smother out the oxidizer by replacing it with another gas (such as opening a can of N2), or cool the area down quickly (such as firing off nanofrost - or even a fire extinguisher).
Decontamination
Let's go back to that greyshirt-canister scenario, and change the gas: Instead of plasma in the main hall, let's say he opened a can of nitrous oxide. Nitrous Oxide, if you'll recall, is nicknamed sleeping gas - if inhaled in large enough quantities, you'll be laying down asleep before you realise it.
Decontamination, however, is fairly straightforward: There's something in the air you don't want, and you want to scrub it out.